How long can galvanized steel resist rust in heavy-duty applications?
Are you specifying materials for a tough environment, worried that the steel components you order will start showing rust in just a few years? A premature corrosion failure can be catastrophic for your product's reputation and your bottom line.
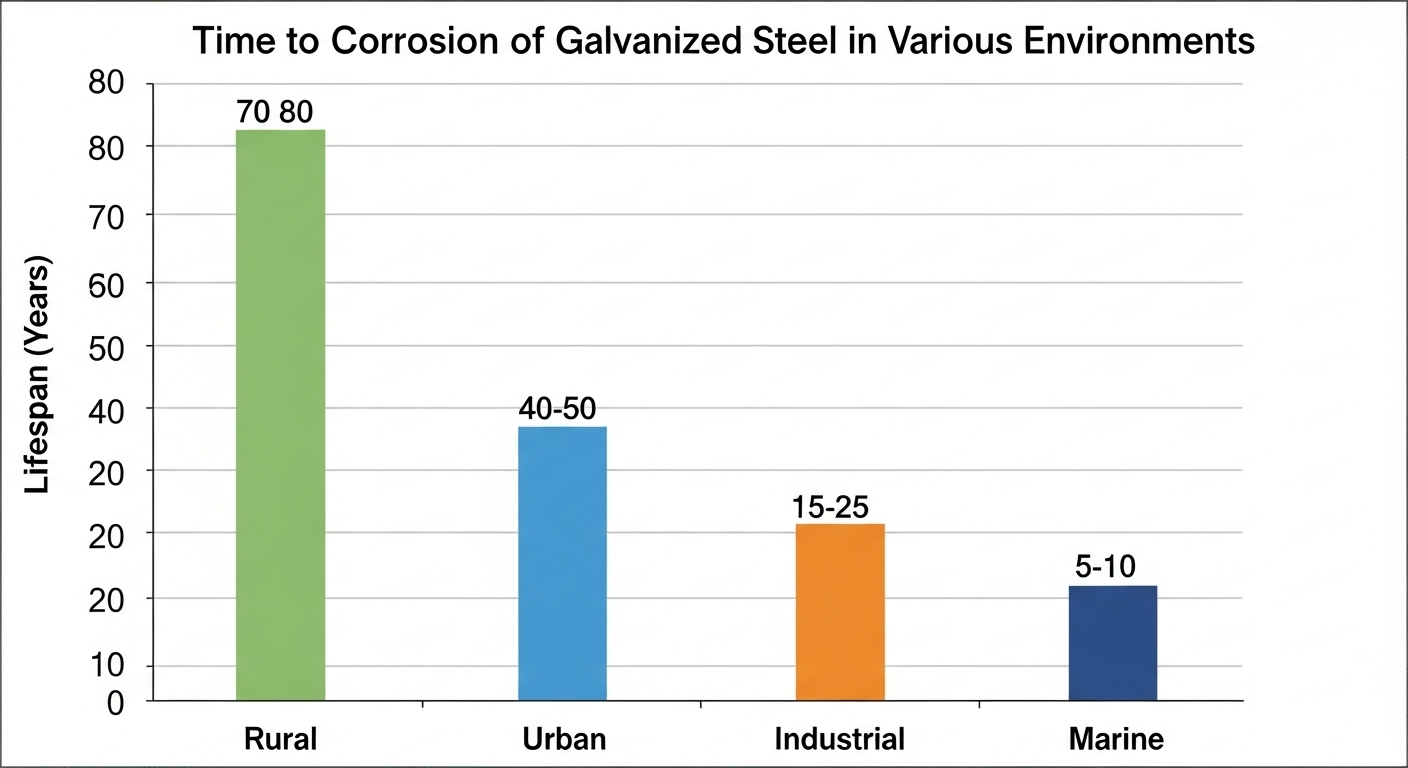
The lifespan of galvanized steel is not a single number; it's a function of the zinc coating's thickness and the severity of the environment. A heavy-duty hot-dip galvanized coating can last over 70 years in a mild rural setting, but that can drop to 5-10 years in an aggressive coastal or industrial zone.
I’ve seen this firsthand, both as a buyer in the U.S. and now, running Prime Metals for over three decades. Clients often ask for a simple guarantee on how long a part will last. The real engineering answer isn't a date on a calendar; it's in understanding the science of how zinc protects steel and the real-world factors that consume that protection. Let’s break it down so you can make an informed choice.
What is galvanizing and why does it protect steel?
Are you thinking of galvanizing as just a simple layer of grey paint on steel? That's a common mistake that leads to underestimating its power, and choosing the wrong material for a tough job.
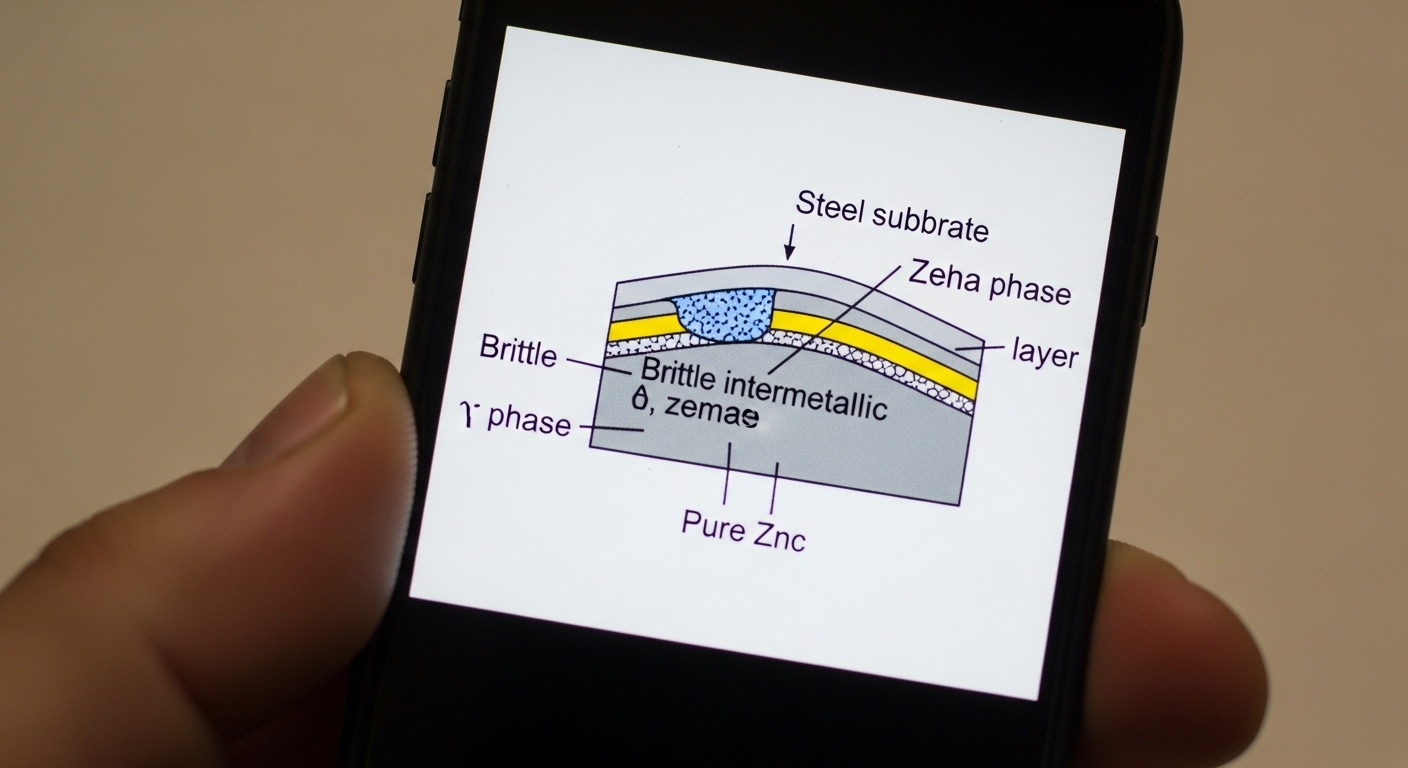
Galvanizing is a process of bonding a layer of zinc to a steel surface. This zinc coating protects the underlying steel in three distinct ways: as a physical barrier, as a sacrificial anode (cathodic protection), and by forming a tough, non-reactive patina over time.
The Physical Barrier
At its most basic level, the zinc coating seals the steel off from the atmosphere. It creates a tough, adherent shield that prevents oxygen and moisture—the key ingredients for rust (iron oxide)—from ever reaching the surface of the steel. This is its first and simplest line of defense.
The Sacrificial Protection
This is where galvanizing truly outperforms paint. Zinc is more electrochemically active than iron. This means when the coating is scratched or damaged, the surrounding zinc will corrode first, sacrificing itself to protect the exposed steel. This "cathodic protection," as explained in detail by sources like Wikipedia on Galvanization, is a powerful, active defense that continues to work even when the barrier is breached.
The Protective Patina
Over time, the zinc coating reacts with the environment to form a thin, hard, and stable layer of zinc carbonate. This layer, known as the patina, is very dense and does not wash away easily. It dramatically slows down the corrosion rate of the zinc itself, acting as a secondary barrier and greatly extending the service life of the protection.
How is zinc coating thickness measured and what do the codes mean?
Are you confused by terms like "G90" or "G60" on a material spec sheet? Choosing the wrong code can mean you are getting a part with only half the protective lifespan you were expecting.
Galvanized coating thickness is most commonly specified by its weight per unit area, using codes defined by standards like ASTM A653. A "G90" coating means there is a total of 0.90 ounces of zinc per square foot of steel surface, which directly corresponds to a thicker, more durable coating than G60 or G30.
Understanding G-Codes
For pre-galvanized sheet metal, the G-code system is standard. The number refers to the total weight of zinc on both sides of the sheet. This means a thicker coating will have a higher G-number and a longer life.
| Designation | Total Zinc (oz/ft²) | Approx. Thickness (mils per side) | Typical Application |
|---|---|---|---|
| G30 | 0.30 | 0.26 | Indoor use, appliances, minimal corrosion risk. |
| G60 | 0.60 | 0.51 | Mild outdoor use, roofing, some construction uses. |
| G90 | 0.90 | 0.76 | Standard for outdoor use, good corrosion resistance. |
| G185 | 1.85 | 1.57 | Culverts, very high corrosion environments. |
Hot-Dip Galvanizing Thickness
For parts that are galvanized after fabrication (post-galvanized), like our custom casting parts or weldments, the thickness is much greater. This process, governed by standards like ASTM A123, specifies minimum coating thicknesses based on the steel's thickness. This often results in coatings 3 to 5 times thicker than a G90 sheet, providing maximum lifespan.
How We Verify Coating Quality
We don't take it on faith. As part of our ISO 9001 certified process, we verify coating thickness using calibrated magnetic gauges. For critical projects, we can also provide lab analysis and salt spray testing reports to certify the coating's performance and adherence to the specification you require.
What environmental factors most aggressively attack galvanized steel?
Do you assume a part that lasts for 50 years in Arizona will also last for 50 years on the Gulf Coast? This is a dangerous and costly assumption that can lead to premature failure in the field.
The service life of galvanized steel is consumed most quickly by moisture, salt (chlorides), and industrial pollutants (sulfur compounds). The combination of these factors in coastal marine or heavy industrial zones creates the most corrosive environments.
Rural / Dry Environments
In an area with low rainfall, low humidity, and no industrial or marine influence, the zinc coating corrodes extremely slowly. The protective patina remains stable, and lifespans of 70-100+ years are common for a heavy coating.
Urban / Temperate Environments
In cities and suburban areas, higher moisture levels and mild pollutants from traffic (sulfur and nitrogen oxides) create a more corrosive environment. This can reduce the lifespan by 20-40% compared to a rural setting.
Industrial Environments
Manufacturing zones with high levels of sulfur dioxide and other airborne pollutants create an acidic environment. When this combines with moisture, it forms acid rain, which aggressively attacks and strips away the zinc coating, dramatically shortening the part's life.
Marine / Coastal Environments
This is often the most severe environment. The combination of high humidity and airborne salt spray (chlorides) creates a potent electrolyte that constantly accelerates the sacrificial corrosion of the zinc. Parts here can have a service life that is less than a tenth of what it would be in a rural zone.
Hot-dip vs. electro-galvanizing: Which is right for my application?
Are you aware that "galvanized" can refer to two completely different processes with wildly different outcomes? Specifying "zinc coated" without clarifying the method can result in getting a cosmetic finish when you needed heavy-duty protection.
Hot-dip galvanizing (HDG) immerses the part in molten zinc, creating a thick, metallurgically-bonded coating for maximum corrosion resistance. Electro-galvanizing (zinc plating) applies a very thin, cosmetic layer of zinc using an electric current, suitable only for mild, indoor environments.
The Hot-Dip Galvanizing (HDG) Process
This is the gold standard for heavy-duty applications. After a series of chemical cleaning baths, the steel part is fully submerged in a kettle of molten zinc at around 450°C (840°F). This creates a series of zinc-iron alloy layers with a pure zinc outer layer. The result is a very thick (typically 50-100 microns), tough, and completely continuous coating.
The Electro-galvanizing (Zinc Plating) Process
This is an electroplating process where the part is placed in a zinc salt solution and an electric current is used to deposit a thin layer of pure zinc. The coating is very smooth, bright, and uniform, but also very thin (typically 5-15 microns). It offers minimal protection and is easily scratched. It's often used for small fasteners, brackets, and indoor chassis that will not be exposed to weather. It's an important option for some of our [custom stamping parts](https://standarddie.com/blog/what-are-the-benefits-of-custom-metal-stamping/).
| Feature | Hot-Dip Galvanizing (HDG) | Electro-Galvanizing (Zinc Plating) |
|---|---|---|
| Thickness | Very Thick (50-100+ μm) | Very Thin (5-15 μm) |
| Protection | Maximum, heavy-duty, outdoor | Minimal, cosmetic, indoor |
| Appearance | Dull gray, crystalline | Bright, shiny, smooth |
| Cost | Higher | Lower |
| Best For | Structural steel, outdoor hardware | Small fasteners, indoor brackets |
How should I design my parts to be galvanized effectively?
Did you know that a poor design can make it impossible to properly galvanize a part? Failing to account for the process can lead to uncoated spots, part distortion, or even safety hazards at the galvanizing plant.
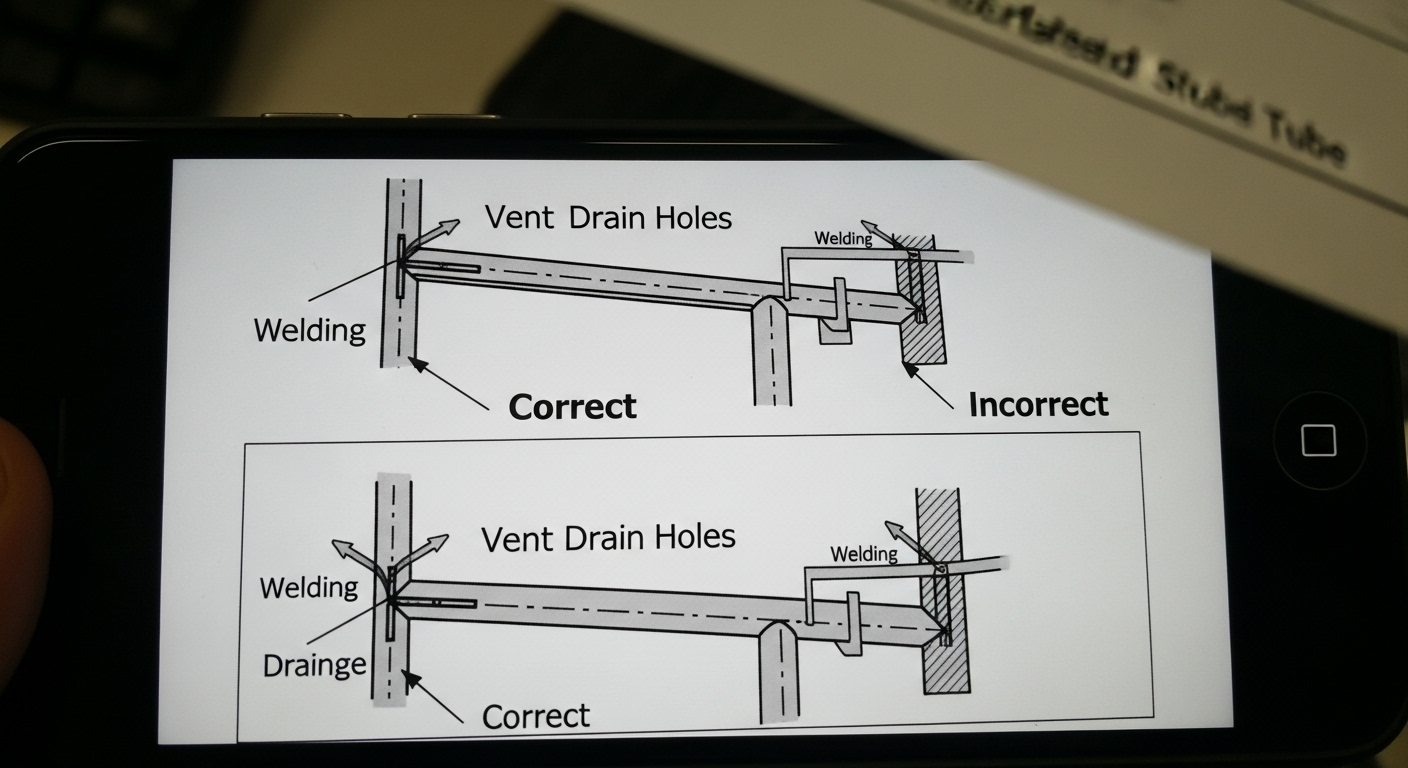
For a part to be successfully hot-dip galvanized, your design must include properly sized and located vent and drain holes for any hollow sections. This allows molten zinc to flow freely and prevents trapped, superheated air from causing explosions.
The Critical Need for Venting and Draining
When a sealed hollow structure is submerged in 450°C zinc, any trapped moisture flashes to steam and expands over 1,700 times its volume, causing a violent explosion. To prevent this, every enclosed section must have holes to allow air and cleaning chemicals to escape and molten zinc to enter and drain out completely, ensuring 100% coating coverage inside and out.
Minimizing Warpage and Distortion
The intense heat of the galvanizing kettle can cause thin, unsupported steel sections to warp, much like a cookie sheet in a hot oven. When we review your design, we will look for these risks. We can often recommend adding temporary bracing or suggest small design changes to increase rigidity and ensure the part maintains its shape.
Considerations for Threaded Parts
The thick coating of HDG will clog up standard threads. For parts with threaded features, this must be planned for. We can either cut the threads after galvanizing (touching up the bare steel with a zinc-rich spray) or use oversized tapped holes and undersized external threads so that they fit correctly after the coating is applied.
About the Author
My name is Kevin. My career started in the US, sourcing heavy industrial components and learning the painful lessons of premature material failure. Since founding Prime Metals in 1993, I have built our factory on the principle of proactive engineering. We work with our customers to select the right materials and processes, ensuring the parts we produce are not only made to print, but are also fit for purpose in their intended service environment.
Frequently Asked Questions (FAQs)
What is "white rust"?
White rust (or wet storage stain) is a white, powdery surface deposit that can form on newly galvanized surfaces when they are nested or stacked tightly in wet, poorly ventilated conditions. It is a surface zinc oxide/hydroxide and is generally not harmful to the coating's lifespan, but it is unsightly.
Can you weld galvanized steel?
Yes, but it requires special precautions. The zinc coating must be ground away from the weld area first. Welding on zinc releases zinc oxide fumes, which are hazardous to inhale, so proper ventilation and respiratory protection are mandatory.
Can galvanized steel be painted or powder coated?
Yes, this is called a duplex system and offers exceptional corrosion resistance. The galvanized surface must be properly prepared first, which usually involves a light abrasive sweep blast and the application of a specific wash primer to ensure the paint adheres correctly.
How does galvanized steel compare to stainless steel?
Galvanized steel is a carbon steel with a protective zinc coating. Stainless steel is an alloy of iron, chromium, and often nickel, which is inherently corrosion-resistant throughout its entire thickness. Galvanized steel is typically much less expensive upfront, but if the coating is compromised, the base steel can rust. Stainless steel offers superior corrosion resistance, especially in chemical environments, but at a significantly higher cost.
Get the Right Protection for Your Project
Choosing the right corrosion protection is a critical engineering decision that impacts the safety, reliability, and lifespan of your product. Don't leave it to chance.
Let our team help you navigate the options and select the most effective and economical solution for your heavy-duty application.